Water hardness is the content of calcium and magnesium ions in water. It is expressed in milligrams of calcium carbonate per 1 liter of water. Water hardness has a negative effect on some farmed animals. For most freshwater fish, the recommended hardness is 20-25 mg/l. If there is a lack of hardness in the water, quicklime is used.
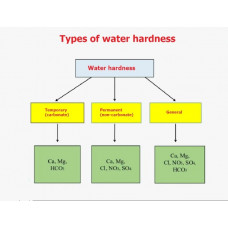
Water with a high salt content is called hard water; water with a low salt content is called soft water. The term "hard" in relation to water was historically formed because of the properties of fabrics after washing them with soap based on fatty acids - fabrics washed in hard water are harder to the touch. This phenomenon is explained, on the one hand, by the sorption by the fabric of calcium and magnesium salts of fatty acids formed during the washing process at the macro level. On the other hand, fabric fibers have ion-exchange properties and, as a consequence, the property to sorb multivalent cations - at the molecular level. A distinction is made between temporary (carbonate) hardness, caused by calcium and magnesium hydrocarbonates Ca(HCO3)2; Mg(HCO3)2, and permanent (non-carbonate) hardness, caused by the presence of other salts that are not released by boiling water: mainly Ca and Mg sulfates and chlorides (CaSO4, CaCl2, MgSO4, MgCl2).
Hard water dries the skin when washing, it does not lather well when using soap. The use of hard water causes scaling on the walls of boilers, pipes, etc. At the same time, the use of too soft water can lead to corrosion of pipes, because in this case there is no acid-alkaline buffering, which is provided by hydrocarbonate (temporary) hardness. The taste of natural drinking water, e.g. spring water, is determined precisely by the presence and ratio of different hardness salts.
The hardness of natural waters can vary over a wide range and is variable throughout the year. Hardness increases due to evaporation, decreases during the rainy season and during snow and ice melt.
The concentration of calcium and magnesium cations in water is used to express water hardness numerically. The recommended SI unit for measuring concentration is mol per cubic meter (mol/m³), but in practice, degrees of hardness and milligram-equivalents per liter (mg-eq/L) are used to measure hardness. Different countries used (and sometimes still use) different out-of-system units - degrees of hardness. According to the value of total hardness, water is distinguished as soft water (up to 2 °J), medium hard water (2-10 °J) and hard water (more than 10 °J).
Water hardness varies widely: from 0.1-0.2 mmol eq/l in rivers and lakes located in the taiga and tundra zones to 80-100 mmol eq/l and more in groundwater, seas and oceans. A distinction is made between soft water (total hardness up to 2 mmol eq/l), medium hardness (2-10 mmol eq/l) and hard water (more than 10 mmol eq/l). In surface water sources, where carbonate hardness prevails (70-80% of total hardness) and magnesium hardness usually does not exceed 30%, rarely 60% of total hardness, water hardness reaches its highest value at the end of winter and its lowest value during the flood period.
Water hardness
Tags: water hardness